
- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
BABIO Secures US FDA DX (k) Clearance ad De Viris Illustribus Transport Kit (Non inactivating)
2025-10-10
BABIO Secures US FDA DX (k) Clearance ad De Viris Illustribus Transport Kit (Non inactivating)
Jinan, China - October 2025 – Jinan Babio Biotechnology Co., Ltd.proudly denuntiat suumBabio® De Viris Illustribus Transport Kit (Non inactivating)quae publice suscepitFDA 510(k) alvi (K250205). Haec certificatio notabile lapidem in BABIO significat, suumque officium confirmat qualitati globali, saluti ac innovationi in diagnosticis clinicis et systematibus microbiologicis onerariis.
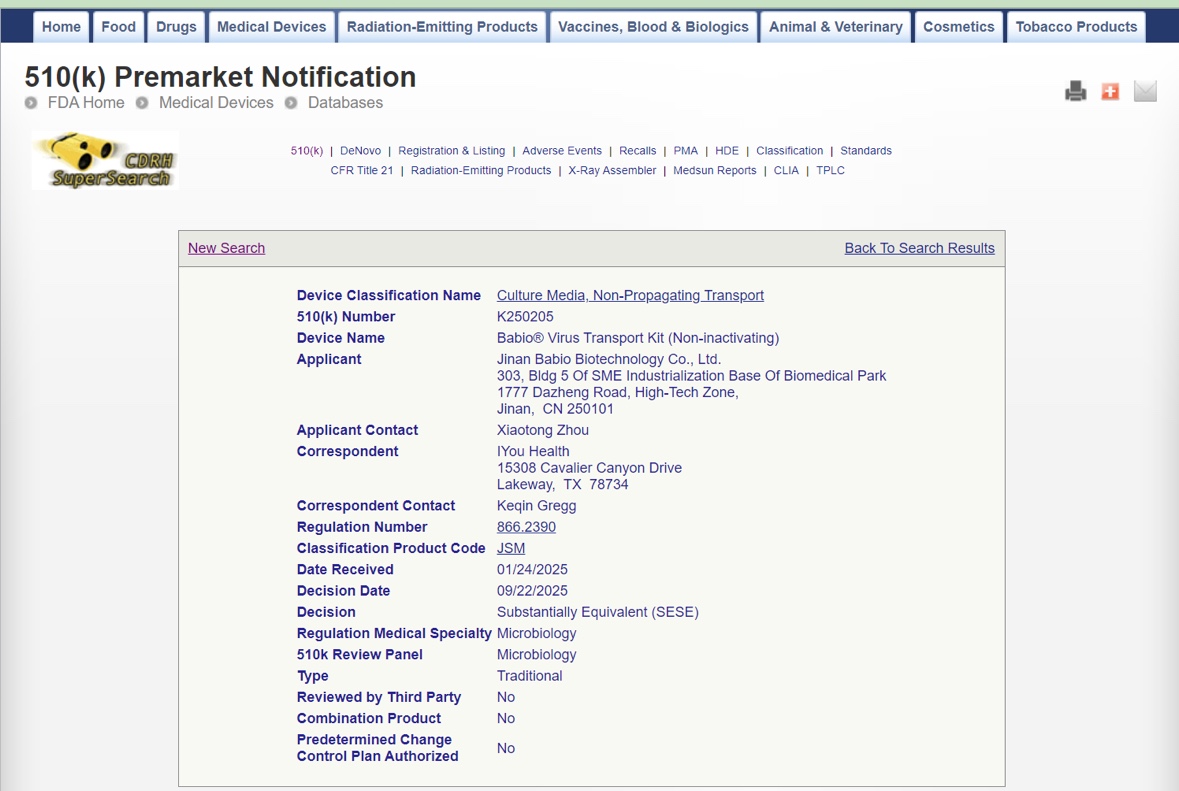
FDA 510(k) alvi deiectio auctore Babio® Virus Transport Kit assecundum substantiam equivalentmachinas in Civitatibus Foederatis legitime venales esse, eius obsequia cum US regulatory ad medicinae machinas confirmandas. Hoc factum demonstrat BABIO validas facultates R&D et praestantiam fabricandi, augendo etiam globalem aemulationem eius invirus transport and specimen collection market.
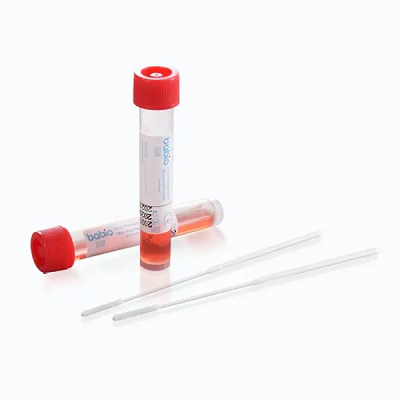
TheBabio® De Viris Illustribus Transport Kit (Non inactivating)ad collectionem et tutam onerariam speciminum clinicorum quae virus continentur, destinatur. Integritatem exempli conservat pro amni experimento utRT-PCR, viral culturaetdiagnostica hypotheticaapta ad valetudinaria, laboratoria et publica valetudine instituta.

BABIO, toducens Chinese manufacturerde diagnostica reagentia, instrumentis onerariis et instrumentis culturae, pergit suam praesentiam dilatareEuropa, Civitates Foederatae, Africa, et Asiae Meridionalispraebens fiduciae solutiones quae signa internationalia conveniunt.
Plura de BABIO certificata producta et innovationes diagnostica, sis visita: https://www.babiocorp.com
#BABIO #FDA510k #VirusTransportKit #MedicalDevices #Diagnostics #BiotechChina #GlobalHealthcare #ClinicalDiagnostics #Microbiology


