
- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
BABIO Virus Transport Kit Earns FDA 510(k) Clearance
2025-10-30
BABIO Virus Transport Kit Earns FDA 510(k) Clearance
Jinan, China - October 2025- BABIO Biotechnologia, nobilis Sinica opifex medicinae diagnostica reagentia, superbe nuntiat suum esseDe Viris Illustribus Transport Kit (Non inactivating)accepitFDA 510(k) alvi (K250205), publice certis cum US regulatory signa.
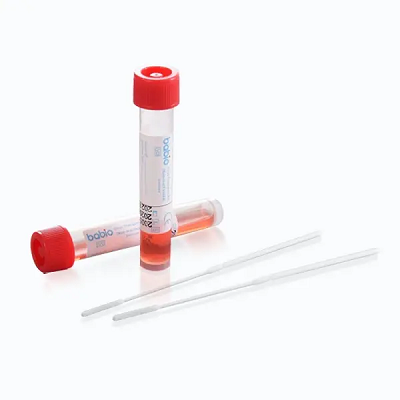
Hoc miliarium subsidia globalis ducis in solutionibus onerariis speciminis clinicae confirmat. Ornamentum machinatum est ad collectionem tutam et stabilem exemplorum viralium, quae applicationes amni sustinent sicutRT-PCR, viral culturaetdiagnostica hypothetica. Is features:
-
✅ Locus temperatus stabilitas
-
✅ Emendatum Hank solutionem cum antibioticis
-
✅ DNase/RNase-liberum fistulae conicae
-
✅ Tutus concursus obiectis cum breakpoint design
Available in pluribus voluminibus repletis (1ml–6ml) et magnitudinum involucrum (20-500 tube), ornamentum est specimen hospitalium, laboratorium et instituta salutis publicae in toto orbe.
Cum a*cotidie productio facultatem 100,000 unitates, BABIO consistent copiam ac auctor cursus sapien. Confisus est inUnited States, Germanyet ultra, BABIO pergit innovationem et fidem in virium onerariis systematibus eripere. Disce plus at https://www.babiocorp.com.
#VirusTransportKit #FDA510k #BABIO #ClinicalDiagnostics #VTM #RT-pcr #GlobalHealthcare #MedicalDevices #specimenCollection


